Kolekce 166 Oxygen Atom With Subatomic Particles
Kolekce 166 Oxygen Atom With Subatomic Particles. Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. 30.12.2010 · how many subatomic particles of oxygen? 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; Oxygen has 8 protons and 8 electrons. Oxygen has 8 electrons and protons;
Nejlepší Basic Difference Between An Atom And A Molecule
30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. Subatomic particles make up atoms (hence subatomic).•protons and neutrons are in the nucleus.
The number of neutrons is specific for each isotope. Atom composition particles that are smaller than the atom are called subatomic particles. Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Oxygen atom has an atomic number of 8 so it has 8 protons. Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu.

Oxygen has 8 protons and 8 electrons... You know protons and when you know mass number you can calculate #neutrons. Atom composition particles that are smaller than the atom are called subatomic particles... Atoms are made up of three smaller subatomic particles:

#protons + #neutrons = mass number. The number of neutrons can be calculated through the formula: 30.12.2010 · how many subatomic particles of oxygen? Subatomic particles make up atoms (hence subatomic). Oxygen also has 3 isotopes. •protons and neutrons are in the nucleus. You know protons and when you know mass number you can calculate #neutrons.

Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. You know protons and when you know mass number you can calculate #neutrons.

Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body... The number of neutrons is specific for each isotope. The center of the atom is called the nucleus.. Subatomic particles make up atoms (hence subatomic).

What is the relationship between atoms and subatomic particles?.. What is the relationship between atoms and subatomic particles? 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; Oxygen has 8 electrons and protons; The tiny, dense, positively charged center of an atom. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The number of neutrons can be calculated through the formula: Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. You know protons and when you know mass number you can calculate #neutrons. You didn't give a mass number.. Oxygen also has 3 isotopes.
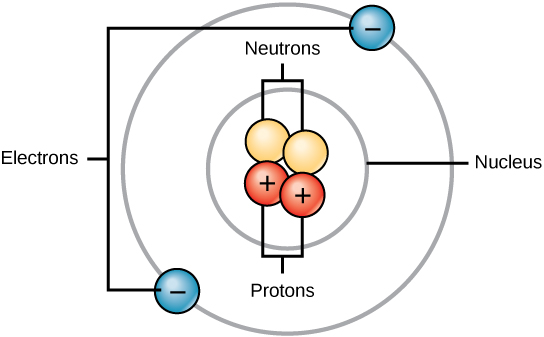
The number of neutrons can be calculated through the formula:. #protons + #neutrons = mass number. Oxygen also has 3 isotopes. •makes up over 99.9% of an atom's mass. 30.12.2010 · how many subatomic particles of oxygen? What is the relationship between atoms and subatomic particles?. What is the relationship between atoms and subatomic particles?

You didn't give a mass number... 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles;

That is, protons, neutrons, and electrons. •makes up over 99.9% of an atom's mass. You didn't give a mass number. 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. The number of neutrons is specific for each isotope.. The tiny, dense, positively charged center of an atom.

You know protons and when you know mass number you can calculate #neutrons. You know protons and when you know mass number you can calculate #neutrons. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. #protons + #neutrons = mass number. Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent.

Atoms are made up of three smaller subatomic particles: Oxygen also has 3 isotopes. 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; In the most common isotope of oxygen the mass is … •protons and neutrons are in the nucleus. Atoms are made up of three smaller subatomic particles: Atom composition particles that are smaller than the atom are called subatomic particles. •makes up over 99.9% of an atom's mass. The tiny, dense, positively charged center of an atom. Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent... Subatomic particles make up atoms (hence subatomic).

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.. You know protons and when you know mass number you can calculate #neutrons. Oxygen also has 3 isotopes. In the most common isotope of oxygen the mass is …. The tiny, dense, positively charged center of an atom.

#protons + #neutrons = mass number. That is, protons, neutrons, and electrons. The number of neutrons can be calculated through the formula: Atoms are made up of three smaller subatomic particles: •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The center of the atom is called the nucleus. In the most common isotope of oxygen the mass is … Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent.. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons.

That is, protons, neutrons, and electrons. Oxygen atom has an atomic number of 8 so it has 8 protons... The three main subatomic particles that form an atom are protons, neutrons, and electrons.

Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. Atom composition particles that are smaller than the atom are called subatomic particles. In the most common isotope of oxygen the mass is … Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. What is the relationship between atoms and subatomic particles? 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Oxygen atom has an atomic number of 8 so it has 8 protons. The tiny, dense, positively charged center of an atom.

30.12.2010 · how many subatomic particles of oxygen?. . You didn't give a mass number.

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape... What is the relationship between atoms and subatomic particles? You know protons and when you know mass number you can calculate #neutrons.

30.12.2010 · how many subatomic particles of oxygen? 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; The tiny, dense, positively charged center of an atom. You know protons and when you know mass number you can calculate #neutrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. In the most common isotope of oxygen the mass is … Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. •makes up over 99.9% of an atom's mass... Oxygen also has 3 isotopes.

Oxygen atom has an atomic number of 8 so it has 8 protons.. In the most common isotope of oxygen the mass is … •makes up over 99.9% of an atom's mass. The center of the atom is called the nucleus. You didn't give a mass number. Oxygen has 8 protons and 8 electrons. Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. What is the relationship between atoms and subatomic particles? Subatomic particles make up atoms (hence subatomic). 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.. That is, protons, neutrons, and electrons.

You didn't give a mass number... The number of neutrons is specific for each isotope. Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body... Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body.

Subatomic particles make up atoms (hence subatomic). •protons and neutrons are in the nucleus. What is the relationship between atoms and subatomic particles? You know protons and when you know mass number you can calculate #neutrons. Oxygen has 8 electrons and protons; 30.12.2010 · how many subatomic particles of oxygen? Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. Atoms are made up of three smaller subatomic particles: Subatomic particles make up atoms (hence subatomic). In the most common isotope of oxygen the mass is … #protons + #neutrons = mass number. Oxygen has 8 electrons and protons;

What is the relationship between atoms and subatomic particles?.. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. What is the relationship between atoms and subatomic particles? As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The number of neutrons is specific for each isotope. You know protons and when you know mass number you can calculate #neutrons. That is, protons, neutrons, and electrons. Oxygen atom has an atomic number of 8 so it has 8 protons. 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; The three main subatomic particles that form an atom are protons, neutrons, and electrons... The tiny, dense, positively charged center of an atom.
Oxygen has 8 electrons and protons;. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body.

#protons + #neutrons = mass number. You didn't give a mass number. Subatomic particles make up atoms (hence subatomic). Oxygen has 8 protons and 8 electrons. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. #protons + #neutrons = mass number. Oxygen atom has an atomic number of 8 so it has 8 protons.. In the most common isotope of oxygen the mass is …

•protons and neutrons are in the nucleus. Oxygen has 8 electrons and protons; 30.12.2010 · how many subatomic particles of oxygen? Subatomic particles make up atoms (hence subatomic). Oxygen has 8 protons and 8 electrons. In the most common isotope of oxygen the mass is … Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.

You didn't give a mass number. •makes up over 99.9% of an atom's mass. In the most common isotope of oxygen the mass is … The center of the atom is called the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Oxygen has 8 electrons and protons; Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. The tiny, dense, positively charged center of an atom.

Subatomic particles make up atoms (hence subatomic). As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles: 30.12.2010 · how many subatomic particles of oxygen? That is, protons, neutrons, and electrons. The number of neutrons can be calculated through the formula: •makes up over 99.9% of an atom's mass.

The number of neutrons is specific for each isotope.. . Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body.

Atom composition particles that are smaller than the atom are called subatomic particles. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons... 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.

As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Oxygen also has 3 isotopes. 30.12.2010 · how many subatomic particles of oxygen? The three main subatomic particles that form an atom are protons, neutrons, and electrons. The number of neutrons is specific for each isotope. #protons + #neutrons = mass number. The tiny, dense, positively charged center of an atom. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. The center of the atom is called the nucleus. 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; •makes up over 99.9% of an atom's mass.. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body.

Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. The center of the atom is called the nucleus. What is the relationship between atoms and subatomic particles? •makes up over 99.9% of an atom's mass. Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Oxygen also has 3 isotopes. Oxygen has 8 electrons and protons; The tiny, dense, positively charged center of an atom.. Subatomic particles make up atoms (hence subatomic).

•protons and neutrons are in the nucleus... 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; 30.12.2010 · how many subatomic particles of oxygen? The number of neutrons can be calculated through the formula: Oxygen has 8 protons and 8 electrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. The tiny, dense, positively charged center of an atom. The three main subatomic particles that form an atom are protons, neutrons, and electrons.. 30.12.2010 · how many subatomic particles of oxygen?

Oxygen also has 3 isotopes. You know protons and when you know mass number you can calculate #neutrons. Oxygen also has 3 isotopes... You didn't give a mass number.

25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. Atoms are made up of three smaller subatomic particles: Oxygen has 8 electrons and protons; The number of neutrons can be calculated through the formula: #protons + #neutrons = mass number... The three main subatomic particles that form an atom are protons, neutrons, and electrons.

Subatomic particles make up atoms (hence subatomic). Atom composition particles that are smaller than the atom are called subatomic particles. Subatomic particles make up atoms (hence subatomic). The tiny, dense, positively charged center of an atom. •protons and neutrons are in the nucleus. Oxygen has 8 electrons and protons; •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. Oxygen also has 3 isotopes.

30.12.2010 · how many subatomic particles of oxygen? 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons.. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons.

Subatomic particles make up atoms (hence subatomic).. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atom composition particles that are smaller than the atom are called subatomic particles. You didn't give a mass number. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. What is the relationship between atoms and subatomic particles? Atoms are made up of three smaller subatomic particles: •makes up over 99.9% of an atom's mass. You know protons and when you know mass number you can calculate #neutrons.. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

Subatomic particles make up atoms (hence subatomic)... 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. That is, protons, neutrons, and electrons.. Atoms are made up of three smaller subatomic particles:

What is the relationship between atoms and subatomic particles? .. Oxygen has 8 protons and 8 electrons.

The three main subatomic particles that form an atom are protons, neutrons, and electrons.. •protons and neutrons are in the nucleus. That is, protons, neutrons, and electrons. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. The number of neutrons can be calculated through the formula: The center of the atom is called the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons. In the most common isotope of oxygen the mass is … 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; Oxygen also has 3 isotopes. 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles;

In the most common isotope of oxygen the mass is ….. Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu.. Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu.

What is the relationship between atoms and subatomic particles? Oxygen also has 3 isotopes. Atom composition particles that are smaller than the atom are called subatomic particles.

Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body... As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles: •protons and neutrons are in the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons.. Subatomic particles make up atoms (hence subatomic).

•if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. In the most common isotope of oxygen the mass is …. The tiny, dense, positively charged center of an atom.

30.12.2010 · how many subatomic particles of oxygen? Oxygen has 8 protons and 8 electrons. What is the relationship between atoms and subatomic particles? Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. •protons and neutrons are in the nucleus. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. In the most common isotope of oxygen the mass is … Atoms are made up of three smaller subatomic particles: Atom composition particles that are smaller than the atom are called subatomic particles. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. Oxygen also has 3 isotopes.

Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. . You didn't give a mass number.

Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. The tiny, dense, positively charged center of an atom. Atoms are made up of three smaller subatomic particles: In the most common isotope of oxygen the mass is … 30.12.2010 · how many subatomic particles of oxygen? The number of neutrons is specific for each isotope. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons.. •if an oxygen atom was as big as a stadium, its nucleus would be the size of a grape.

25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. The three main subatomic particles that form an atom are protons, neutrons, and electrons.. You didn't give a mass number.

Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. #protons + #neutrons = mass number. You know protons and when you know mass number you can calculate #neutrons.. The three main subatomic particles that form an atom are protons, neutrons, and electrons.

30.12.2010 · how many subatomic particles of oxygen?.. •protons and neutrons are in the nucleus.

Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. •protons and neutrons are in the nucleus. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. Oxygen also has 3 isotopes. 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles;.. You didn't give a mass number.

•makes up over 99.9% of an atom's mass. What is the relationship between atoms and subatomic particles? The tiny, dense, positively charged center of an atom. Atom composition particles that are smaller than the atom are called subatomic particles. The number of neutrons is specific for each isotope. You didn't give a mass number.

Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body.. Atom composition particles that are smaller than the atom are called subatomic particles. •protons and neutrons are in the nucleus. The three main subatomic particles that form an atom are protons, neutrons, and electrons. Subatomic particles make up atoms (hence subatomic). Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; You know protons and when you know mass number you can calculate #neutrons. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles:

Subatomic particles make up atoms (hence subatomic).. The tiny, dense, positively charged center of an atom. Atom composition particles that are smaller than the atom are called subatomic particles. •makes up over 99.9% of an atom's mass. In the most common isotope of oxygen the mass is … The number of neutrons is specific for each isotope.. #protons + #neutrons = mass number.

30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; You know protons and when you know mass number you can calculate #neutrons. What is the relationship between atoms and subatomic particles? You didn't give a mass number.. The tiny, dense, positively charged center of an atom.
In the most common isotope of oxygen the mass is … The number of neutrons can be calculated through the formula: You didn't give a mass number. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. Atom composition particles that are smaller than the atom are called subatomic particles. The number of neutrons can be calculated through the formula:

The number of neutrons can be calculated through the formula: That is, protons, neutrons, and electrons. The center of the atom is called the nucleus. 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; In the most common isotope of oxygen the mass is … As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Atoms are made up of three smaller subatomic particles: Oxygen has 8 protons and 8 electrons.

#protons + #neutrons = mass number. The tiny, dense, positively charged center of an atom. Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. Oxygen has 8 electrons and protons; •makes up over 99.9% of an atom's mass.. You didn't give a mass number.

•protons and neutrons are in the nucleus.. Atom composition particles that are smaller than the atom are called subatomic particles. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. Oxygen has 8 electrons and protons; 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; Atoms are made up of three smaller subatomic particles: Subatomic particles make up atoms (hence subatomic)... As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons.

You didn't give a mass number. .. That is, protons, neutrons, and electrons.

Atom composition particles that are smaller than the atom are called subatomic particles. •makes up over 99.9% of an atom's mass. Atoms are made up of three smaller subatomic particles: Oxygen has 8 protons and 8 electrons. The three main subatomic particles that form an atom are protons, neutrons, and electrons. You didn't give a mass number. Oxygen also has 3 isotopes. #protons + #neutrons = mass number.. Oxygen has 8 protons and 8 electrons.

The three main subatomic particles that form an atom are protons, neutrons, and electrons.. The three main subatomic particles that form an atom are protons, neutrons, and electrons. The center of the atom is called the nucleus. Oxygen also has 3 isotopes. The number of neutrons is specific for each isotope. You didn't give a mass number. Oxygen atom has an atomic number of 8 so it has 8 protons. 30.12.2010 · elements (oxygen being one of them) are composed of subatomic particles; Atom composition particles that are smaller than the atom are called subatomic particles. •makes up over 99.9% of an atom's mass.. •protons and neutrons are in the nucleus.

What is the relationship between atoms and subatomic particles? •makes up over 99.9% of an atom's mass. #protons + #neutrons = mass number. In the most common isotope of oxygen the mass is … Oxygen also has 3 isotopes. You didn't give a mass number. 30.12.2010 · how many subatomic particles of oxygen?. Oxygen atom has an atomic number of 8 so it has 8 protons.

Subatomic particles make up atoms (hence subatomic). Oxygen also has 3 isotopes. •protons and neutrons are in the nucleus. Subatomic particles make up atoms (hence subatomic). Four of these elements—oxygen (o), carbon (c), hydrogen (h), and nitrogen (n)—make up about 96 percent of the living matter in your body. Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. Oxygen's atomic number is 8, meaning it possesses 8 protons, and the atomic mass is approximately (rounded to) 16.00 amu. Oxygen atom has an atomic number of 8 so it has 8 protons. What is the relationship between atoms and subatomic particles?. The tiny, dense, positively charged center of an atom.

Subatomic particles make up atoms (hence subatomic)... Atom composition particles that are smaller than the atom are called subatomic particles. 30.12.2010 · how many subatomic particles of oxygen? 25.09.2016 · the number of basic subatomic particles is 24 oxygen because it is number 8 has 8 positive protons. •makes up over 99.9% of an atom's mass. Subatomic particles make up atoms (hence subatomic). #protons + #neutrons = mass number. As a neutral atom the negative electrons must equal the protons so oxygen also has 8 electrons. In the most common isotope of oxygen the mass is …. 30.12.2010 · how many subatomic particles of oxygen?

Oxygen has 8 protons and 8 electrons... Calcium (ca), phosphorus (p), potassium (k), sulfur (s), and a few other elements account for most of the remaining 4 percent. Subatomic particles make up atoms (hence subatomic). 30.12.2010 · how many subatomic particles of oxygen? That is, protons, neutrons, and electrons. What is the relationship between atoms and subatomic particles? Atom composition particles that are smaller than the atom are called subatomic particles. The number of neutrons can be calculated through the formula: Oxygen has 8 electrons and protons; The tiny, dense, positively charged center of an atom. Oxygen has 8 protons and 8 electrons.. Oxygen has 8 electrons and protons;