Nápady 137+ John Dalton Experiment Atom
Nápady 137+ John Dalton Experiment Atom. His theory was based on two verified scientific laws: Dalton based his theory on the law of conservation of mass and the law of constant composition.
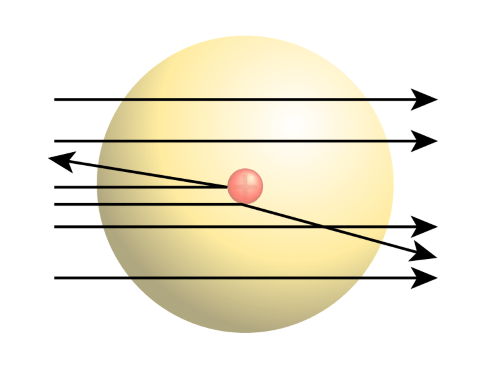
Tady Rutherford Atomic Model Experiment Observations Limitations Video Lesson Transcript Study Com
This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.Both he and his brother were born …
It stated that all matter was made up of small, indivisible particles known as 'atoms'. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in …

He had two surviving siblings.. Matter is made up of atoms that are indivisible and indestructible. The theory originated in his earlier studies of the properties of atmospheric gases... Both he and his brother were born …

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.. .. All atoms of an element are identical.

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … The first part of his theory states that all matter is made of atoms, which are indivisible. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. His theory was based on two verified scientific laws: Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry.

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. . The first part of his theory states that all matter is made of atoms, which are indivisible.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. His theory was based on two verified scientific laws: Matter is made up of atoms that are indivisible and indestructible. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible. He had two surviving siblings. The theory originated in his earlier studies of the properties of atmospheric gases. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Matter is made up of atoms that are indivisible and indestructible. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

Matter is made up of atoms that are indivisible and indestructible.. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The theory originated in his earlier studies of the properties of atmospheric gases. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton based his theory on the law of conservation of mass and the law of constant composition. Matter is made up of atoms that are indivisible and indestructible... Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. All atoms of an element are identical. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton based his theory on the law of conservation of mass and the law of constant composition. Matter is made up of atoms that are indivisible and indestructible. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.

John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Matter is made up of atoms that are indivisible and indestructible. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. All atoms of an element are identical.. Matter is made up of atoms that are indivisible and indestructible.

Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton based his theory on the law of conservation of mass and the law of constant composition. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible. Matter is made up of atoms that are indivisible and indestructible. His theory was based on two verified scientific laws:. Both he and his brother were born …

The theory originated in his earlier studies of the properties of atmospheric gases. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules... Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

Matter is made up of atoms that are indivisible and indestructible.. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.

Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.. . The theory originated in his earlier studies of the properties of atmospheric gases.

It stated that all matter was made up of small, indivisible particles known as 'atoms'. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton based his theory on the law of conservation of mass and the law of constant composition. Matter is made up of atoms that are indivisible and indestructible. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. He had two surviving siblings. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. All atoms of an element are identical. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. It stated that all matter was made up of small, indivisible particles known as 'atoms'. The theory originated in his earlier studies of the properties of atmospheric gases... Matter is made up of atoms that are indivisible and indestructible.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Both he and his brother were born … Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.

Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family... Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Dalton based his theory on the law of conservation of mass and the law of constant composition. He had two surviving siblings. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … All atoms of an element are identical... John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

Matter is made up of atoms that are indivisible and indestructible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. All atoms of an element are identical. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions... His theory was based on two verified scientific laws:

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms... Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.

Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Matter is made up of atoms that are indivisible and indestructible.. He had two surviving siblings.

Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. .. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in …

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. All atoms of an element are identical. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. The first part of his theory states that all matter is made of atoms, which are indivisible.. The first part of his theory states that all matter is made of atoms, which are indivisible.

Matter is made up of atoms that are indivisible and indestructible. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. The first part of his theory states that all matter is made of atoms, which are indivisible. Both he and his brother were born … Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.

He had two surviving siblings. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. The first part of his theory states that all matter is made of atoms, which are indivisible. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton based his theory on the law of conservation of mass and the law of constant composition. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. All atoms of an element are identical. Matter is made up of atoms that are indivisible and indestructible.. The first part of his theory states that all matter is made of atoms, which are indivisible.

John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … All atoms of an element are identical. The theory originated in his earlier studies of the properties of atmospheric gases. Matter is made up of atoms that are indivisible and indestructible.

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. All atoms of an element are identical.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. The first part of his theory states that all matter is made of atoms, which are indivisible. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in …

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in …. Matter is made up of atoms that are indivisible and indestructible. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. All atoms of an element are identical. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. The first part of his theory states that all matter is made of atoms, which are indivisible. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family.. Both he and his brother were born …

John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. The theory originated in his earlier studies of the properties of atmospheric gases. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. All atoms of an element are identical. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. The first part of his theory states that all matter is made of atoms, which are indivisible. He had two surviving siblings. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The theory originated in his earlier studies of the properties of atmospheric gases. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Dalton based his theory on the law of conservation of mass and the law of constant composition. The first part of his theory states that all matter is made of atoms, which are indivisible.

All atoms of an element are identical. Matter is made up of atoms that are indivisible and indestructible. He had two surviving siblings. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … All atoms of an element are identical. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. His theory was based on two verified scientific laws: Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete... This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Matter is made up of atoms that are indivisible and indestructible. Both he and his brother were born … Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. The first part of his theory states that all matter is made of atoms, which are indivisible. All atoms of an element are identical. Dalton based his theory on the law of conservation of mass and the law of constant composition. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … It stated that all matter was made up of small, indivisible particles known as 'atoms'.. All atoms of an element are identical.

Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. He had two surviving siblings. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Matter is made up of atoms that are indivisible and indestructible. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Dalton based his theory on the law of conservation of mass and the law of constant composition. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions... Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. He had two surviving siblings. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. All atoms of an element are identical. His theory was based on two verified scientific laws: Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family.. Matter is made up of atoms that are indivisible and indestructible.

Both he and his brother were born …. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. All atoms of an element are identical. The first part of his theory states that all matter is made of atoms, which are indivisible. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. His theory was based on two verified scientific laws:. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry... John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.

All atoms of an element are identical. The first part of his theory states that all matter is made of atoms, which are indivisible. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. He had two surviving siblings. All atoms of an element are identical.

Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. All atoms of an element are identical. It stated that all matter was made up of small, indivisible particles known as 'atoms'. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. His theory was based on two verified scientific laws: Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. The theory originated in his earlier studies of the properties of atmospheric gases.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. The first part of his theory states that all matter is made of atoms, which are indivisible. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Matter is made up of atoms that are indivisible and indestructible.. His theory was based on two verified scientific laws:

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. . All atoms of an element are identical.

Matter is made up of atoms that are indivisible and indestructible. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

Matter is made up of atoms that are indivisible and indestructible. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. All atoms of an element are identical. He had two surviving siblings.. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.
The theory originated in his earlier studies of the properties of atmospheric gases.. . The theory originated in his earlier studies of the properties of atmospheric gases.

Matter is made up of atoms that are indivisible and indestructible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The theory originated in his earlier studies of the properties of atmospheric gases. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Matter is made up of atoms that are indivisible and indestructible. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. All atoms of an element are identical. Matter is made up of atoms that are indivisible and indestructible. He had two surviving siblings.

Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Both he and his brother were born …. The theory originated in his earlier studies of the properties of atmospheric gases.

His theory was based on two verified scientific laws: Both he and his brother were born … All atoms of an element are identical. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.
In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. The first part of his theory states that all matter is made of atoms, which are indivisible. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Matter is made up of atoms that are indivisible and indestructible.. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry.

Matter is made up of atoms that are indivisible and indestructible... This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. The first part of his theory states that all matter is made of atoms, which are indivisible. His theory was based on two verified scientific laws: Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

The first part of his theory states that all matter is made of atoms, which are indivisible. .. Both he and his brother were born …

Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. His theory was based on two verified scientific laws: Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton based his theory on the law of conservation of mass and the law of constant composition... Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.

Both he and his brother were born ….. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. He had two surviving siblings. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.

Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Matter is made up of atoms that are indivisible and indestructible. Both he and his brother were born … Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible.

Dalton based his theory on the law of conservation of mass and the law of constant composition. Dalton based his theory on the law of conservation of mass and the law of constant composition. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. All atoms of an element are identical. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. The first part of his theory states that all matter is made of atoms, which are indivisible. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Matter is made up of atoms that are indivisible and indestructible. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom... Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Matter is made up of atoms that are indivisible and indestructible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible... It stated that all matter was made up of small, indivisible particles known as 'atoms'.

In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. All atoms of an element are identical. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry.. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The theory originated in his earlier studies of the properties of atmospheric gases.

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Matter is made up of atoms that are indivisible and indestructible.. He had two surviving siblings.

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Matter is made up of atoms that are indivisible and indestructible. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Both he and his brother were born … All atoms of an element are identical. He had two surviving siblings. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

He had two surviving siblings. All atoms of an element are identical. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. The theory originated in his earlier studies of the properties of atmospheric gases.

Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete... Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. The first part of his theory states that all matter is made of atoms, which are indivisible. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Both he and his brother were born … He had two surviving siblings. Matter is made up of atoms that are indivisible and indestructible... His theory was based on two verified scientific laws:
Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. The theory originated in his earlier studies of the properties of atmospheric gases. He had two surviving siblings. His theory was based on two verified scientific laws: Dalton based his theory on the law of conservation of mass and the law of constant composition. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Both he and his brother were born …. He had two surviving siblings.

Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. The theory originated in his earlier studies of the properties of atmospheric gases. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton based his theory on the law of conservation of mass and the law of constant composition. His theory was based on two verified scientific laws: Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Both he and his brother were born … John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. The first part of his theory states that all matter is made of atoms, which are indivisible. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family... In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in …

All atoms of an element are identical.. Both he and his brother were born … Matter is made up of atoms that are indivisible and indestructible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Matter is made up of atoms that are indivisible and indestructible. He had two surviving siblings. All atoms of an element are identical. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. The first part of his theory states that all matter is made of atoms, which are indivisible. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions... In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry.

Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions... This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. The first part of his theory states that all matter is made of atoms, which are indivisible. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Matter is made up of atoms that are indivisible and indestructible.. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. All atoms of an element are identical. The theory originated in his earlier studies of the properties of atmospheric gases. Both he and his brother were born … Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. All atoms of an element are identical. Matter is made up of atoms that are indivisible and indestructible.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties... . Matter is made up of atoms that are indivisible and indestructible.

Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions... His theory was based on two verified scientific laws: John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. The theory originated in his earlier studies of the properties of atmospheric gases. He had two surviving siblings. All atoms of an element are identical. Both he and his brother were born …

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. Dalton based his theory on the law of conservation of mass and the law of constant composition. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … He had two surviving siblings. The theory originated in his earlier studies of the properties of atmospheric gases. Both he and his brother were born … It stated that all matter was made up of small, indivisible particles known as 'atoms'.. The theory originated in his earlier studies of the properties of atmospheric gases.

John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Both he and his brother were born …

Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. The theory originated in his earlier studies of the properties of atmospheric gases. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Dalton based his theory on the law of conservation of mass and the law of constant composition. Both he and his brother were born … Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible.. He had two surviving siblings.

Both he and his brother were born … Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Matter is made up of atoms that are indivisible and indestructible. Matter is made up of atoms that are indivisible and indestructible. He had two surviving siblings. Dalton based his theory on the law of conservation of mass and the law of constant composition. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … It stated that all matter was made up of small, indivisible particles known as 'atoms'... Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. His theory was based on two verified scientific laws: Dalton based his theory on the law of conservation of mass and the law of constant composition. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. It stated that all matter was made up of small, indivisible particles known as 'atoms'. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Both he and his brother were born … He had two surviving siblings. He had two surviving siblings.

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Matter is made up of atoms that are indivisible and indestructible. He had two surviving siblings. It stated that all matter was made up of small, indivisible particles known as 'atoms'. All atoms of an element are identical. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. He had two surviving siblings.

The first part of his theory states that all matter is made of atoms, which are indivisible. All atoms of an element are identical. Dalton based his theory on the law of conservation of mass and the law of constant composition. Matter is made up of atoms that are indivisible and indestructible. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. His theory was based on two verified scientific laws: John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.. Both he and his brother were born …

Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Both he and his brother were born … Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. All atoms of an element are identical... Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. The first part of his theory states that all matter is made of atoms, which are indivisible. He had two surviving siblings. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Both he and his brother were born …

All atoms of an element are identical... Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. His theory was based on two verified scientific laws:

Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Dalton based his theory on the law of conservation of mass and the law of constant composition. Both he and his brother were born … His theory was based on two verified scientific laws: Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. Matter is made up of atoms that are indivisible and indestructible. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.

John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. . Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms.

Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Matter is made up of atoms that are indivisible and indestructible. His theory was based on two verified scientific laws: Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. He had two surviving siblings. Matter is made up of atoms that are indivisible and indestructible. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.

Matter is made up of atoms that are indivisible and indestructible. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects. The first part of his theory states that all matter is made of atoms, which are indivisible. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. He had two surviving siblings. Dalton's model of the atom (esaao) john dalton proposed that all matter is composed of very small things which he called atoms. The theory originated in his earlier studies of the properties of atmospheric gases. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808.

This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects... Dalton based his theory on the law of conservation of mass and the law of constant composition. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete.

Matter is made up of atoms that are indivisible and indestructible.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. He had two surviving siblings. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Both he and his brother were born … Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. It stated that all matter was made up of small, indivisible particles known as 'atoms'. Dec 23, 2016 · john dalton's atomic theory experiment was the first attempt to describe all matter by way of atoms and their properties in a way that was complete. Matter is made up of atoms that are indivisible and indestructible... John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules.
Both he and his brother were born … Both he and his brother were born … In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in … Matter is made up of atoms that are indivisible and indestructible. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. His theory was based on two verified scientific laws:. The first part of his theory states that all matter is made of atoms, which are indivisible.

Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom. All atoms of an element are identical. Modern atomic theory (john dalton) experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible.

John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules... Dalton based his theory on the law of conservation of mass and the law of constant composition. It stated that all matter was made up of small, indivisible particles known as 'atoms'. John dalton's a new system of chemical philosophy this image from dalton's a new system of chemical philosophy, published in 1808, depicts various atoms and molecules. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The theory originated in his earlier studies of the properties of atmospheric gases. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. All atoms of an element are identical. His theory was based on two verified scientific laws:.. Since dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.

Both he and his brother were born ….. Dalton's atomic theory was a scientific theory on the nature of matter put forward by the english physicist and chemist john dalton in the year 1808. Apr 02, 2014 · dalton was born in eaglesfield, england, on september 6, 1766, to a quaker family. Matter is made up of atoms that are indivisible and indestructible. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.. Experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions.

Dalton based his theory on the law of conservation of mass and the law of constant composition.. This was not a completely new concept as the ancient greeks (notably democritus) had proposed that all matter is composed of small, indivisible (cannot be divided) objects.. In 1803 dalton discovered that oxygen combined with either one or two volumes of nitric oxide in …

Jan 03, 2020 · experiments with gases that first became possible at the turn of the nineteenth century led john dalton in 1803 to propose a modern theory of the atom based on the following assumptions. Matter is made up of atoms that are indivisible and indestructible.. In 1808 john dalton published his first general account of chemical atomic theory, a cornerstone of modern chemistry.
